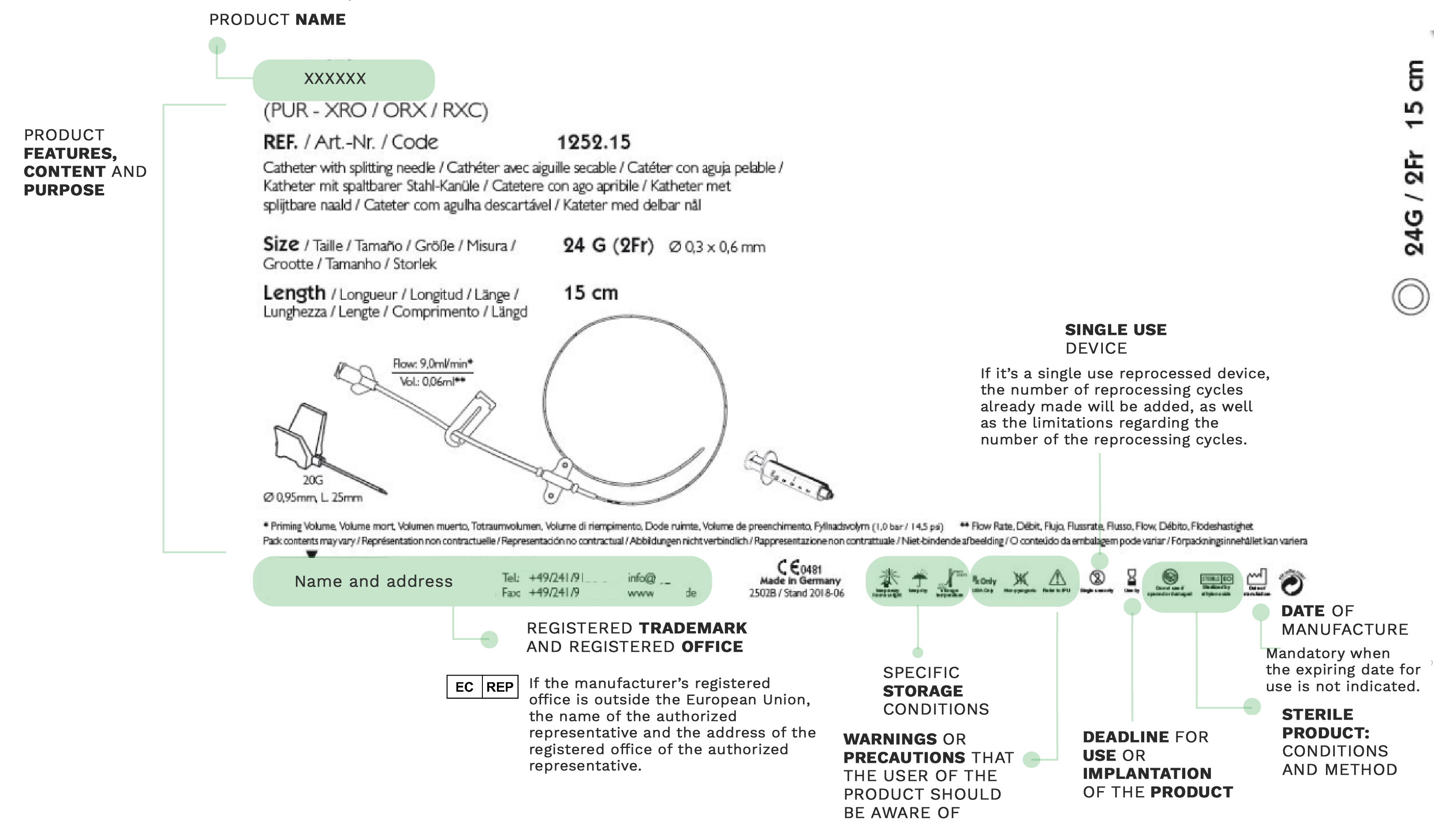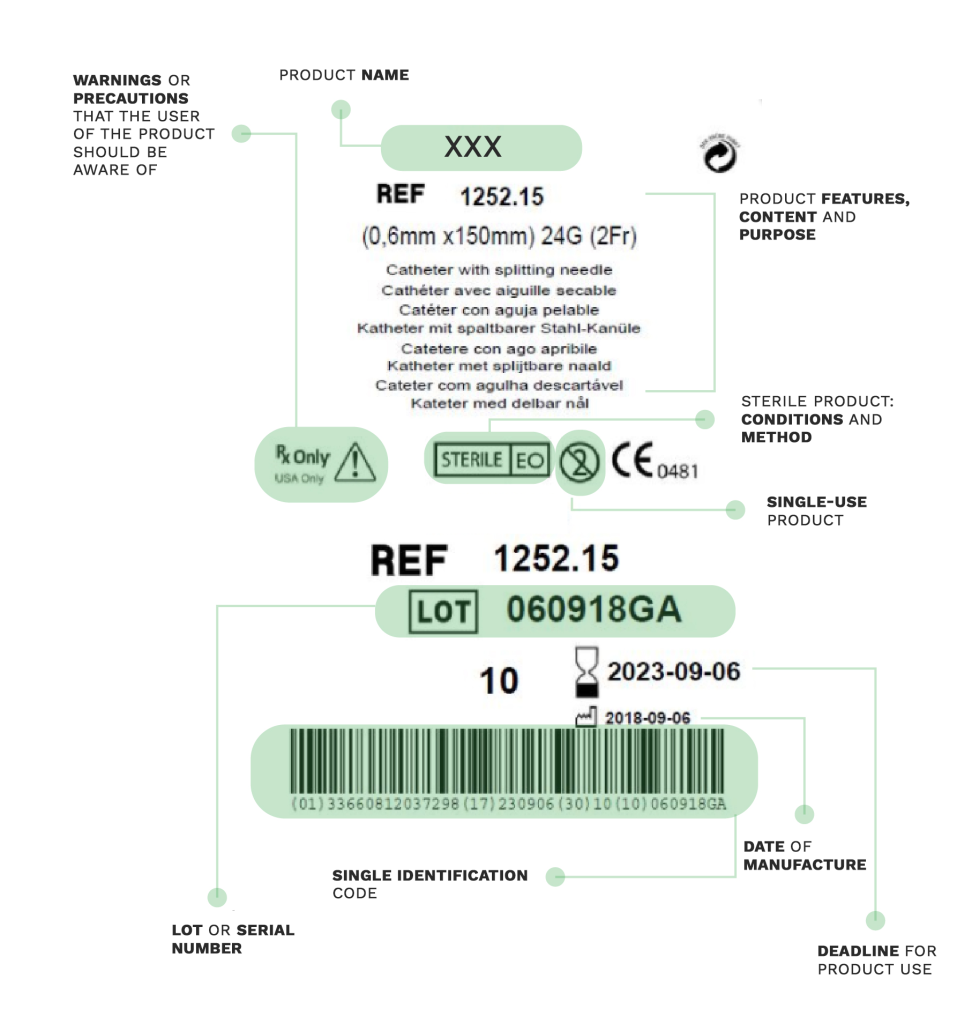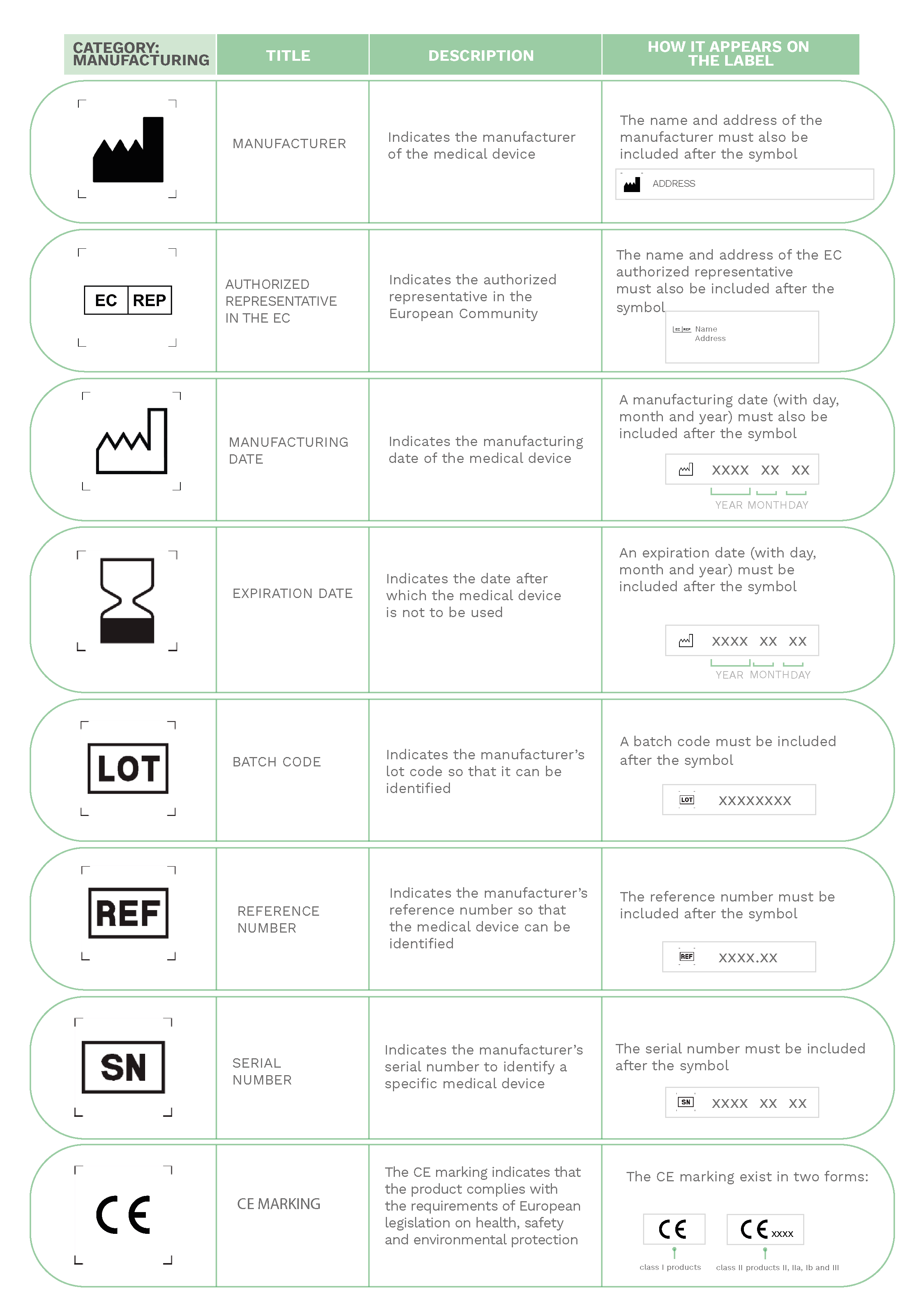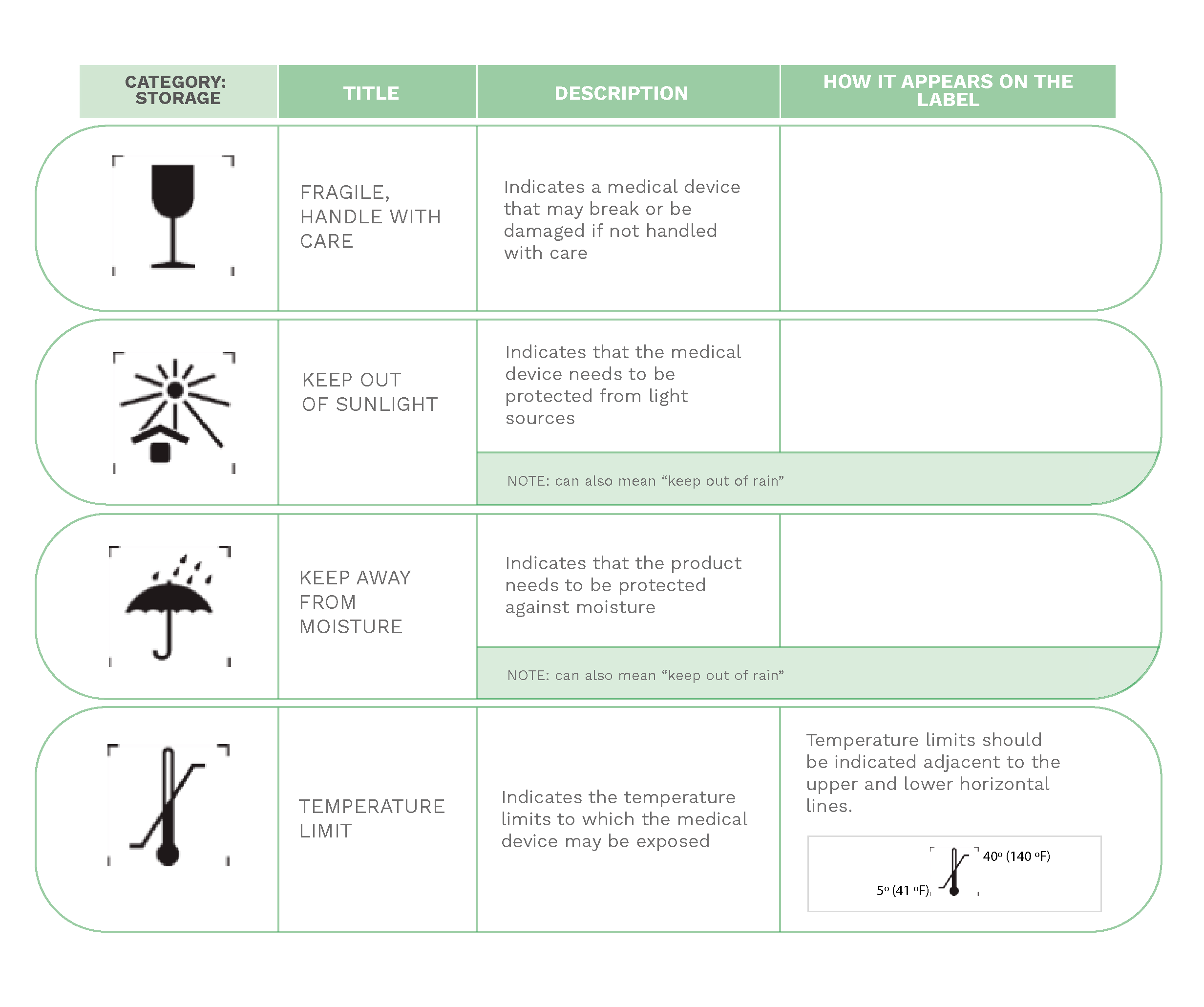
On medical devices labels, manufacturers have to include the necessary information about the equipment that healthcare professionals are going to use for patient care.
Provided on products’ batches or packaging, labels are an essential tool to identify the product and its manufacturer, as well as the information related to the safety and operation of the device.
Proper understanding of the labeling can facilitate the correct use of the device and avoid incorrect application or handling that could cause complications. A misunderstanding could jeopardize the patient’s safety or compromise the proper functioning of the treatment applied.
In other words, knowing how to read and interpret the labeling will facilitate the work of the teams and improve healthcare.
BUT HOW CAN THE INFORMATION ON THE LABELS BE INTERPRETED?
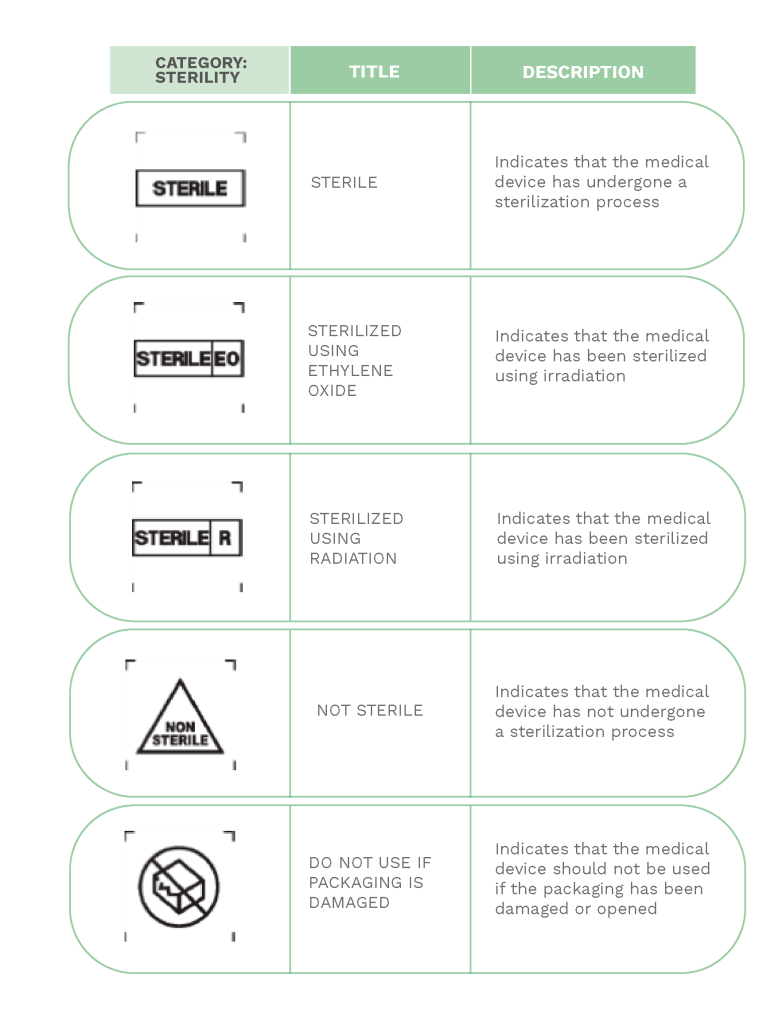
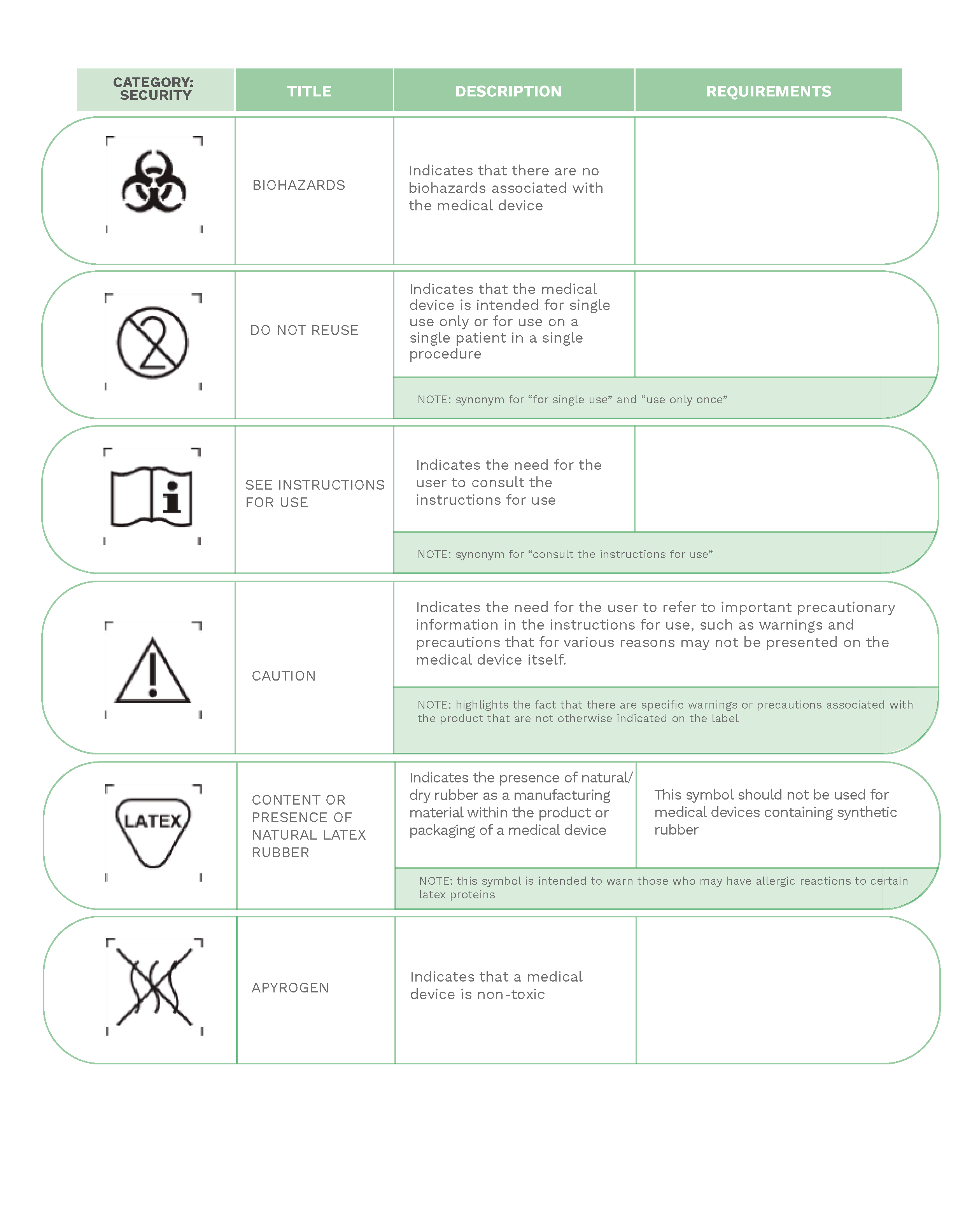
Much of the information provided on labels is expressed through a series of internationally known symbols adapted to harmonized standards or CS standards.
In the following compilation, you will find the most commonly used signs in labeling:
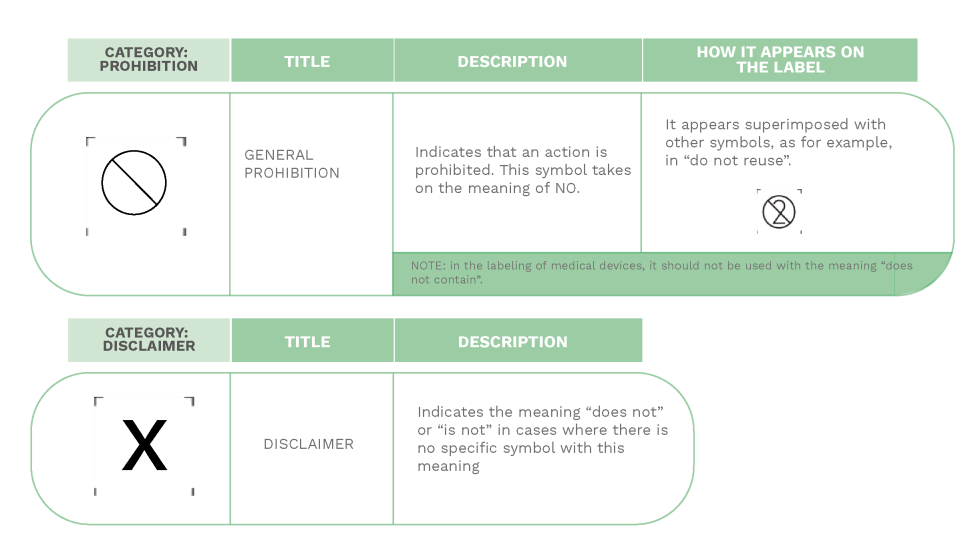
USE OF PROHIBITION AND DISCLAIMER SIGNS ON MEDICAL DEVICES LABELS
In addition to the symbols mentioned above, there are other signs that, in combination with the ones we saw before, can be found on the labels of medical devices.
These two signs (Ø and X) refer to the general prohibition and disclaimer rules. Their use is permitted. However, they are not encouraged (except for the symbol do not reuse which, in this case, is a standardized sign).
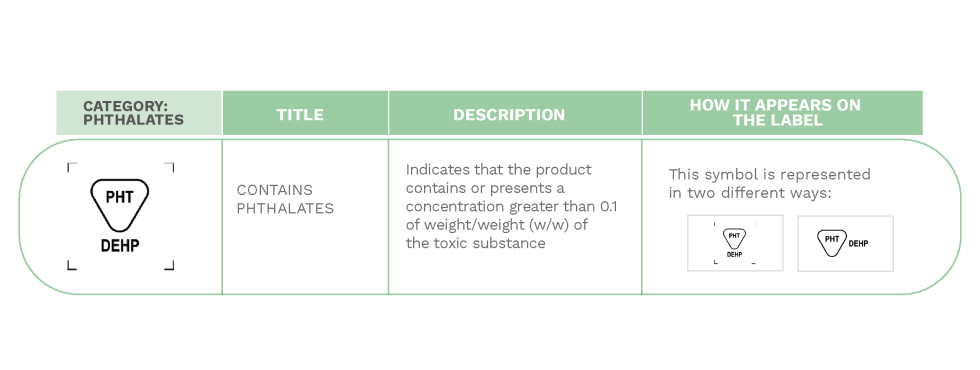
LABELING OF PRODUCTS CONTAINING PHTHALATES ON MEDICAL DEVICES LABELS
Some medical devices have a special distinction on their label. This is because sometimes, in order to change the physical or biological characteristics of a manufacturing material, it is common to add chemicals such as phthalates.
Phthalates are substances considered to be toxic when in contact with the patient’s fluids. Manufacturers must notify healthcare professionals if this type of products is used, including in the instructions for use regarding the residual risks and, if necessary, the appropriate precautionary measures related to the handling. This is especially the case when their use is intended for the treatment of children, pregnant or breastfeeding women and other groups of patients considered vulnerable.
“For this reason, according to European guidelines, in addition to the previous information, all medical devices containing these substances in a concentration greater than 0.1% by weight/weight (w/w), must be clearly identified with the relevant symbol (shown below) on the label of the product itself.”
Complications arising from not knowing the symbols indicated on the labels and not handling, using, and storing the devices according to the information provided on them, can endanger patients’ health. Additionally, failure to comply with these guidelines can lead to legal consequences for the healthcare facility.
If you have any question about the symbols or additional information on the labels of the medical devices you use, do not hesitate to contact your manufacturer to clear up your doubts.
BIBLIOGRAPHY
-
Spanish Association for Standardization (2019). Symbols to be used on labels, labeling and information to be supplied (ISO 12223-1).
-
Spanish Association for Standardization and Certification (2011). Symbols to be used in the labeling of medical devices: Requirements for the labeling of medical devices containing phthalates (UNE- EN 15986).
-
European Parliament and of the Council (2017). European Regulation on Medical Devices (EU – 2017/745).